-
PDF
- Split View
-
Views
-
Cite
Cite
Rita-Josiane Gouesse, Mélanie Lavoie, Elham Dianati, Mike G Wade, Barbara F Hales, Bernard Robaire, Isabelle Plante, Gestational and Lactational Exposure to an Environmentally Relevant Mixture of Brominated Flame Retardants Downregulates Junctional Proteins, Thyroid Hormone Receptor α1 Expression, and the Proliferation-Apoptosis Balance in Mammary Glands Post Puberty, Toxicological Sciences, Volume 171, Issue 1, September 2019, Pages 13–31, https://doi.org/10.1093/toxsci/kfz147
Close - Share Icon Share
Abstract
Mammary gland development requires hormonal regulation during puberty, pregnancy, and lactation. Brominated flame retardants (BFRs) are endocrine disruptors; they are added to consumer products to satisfy flammability standards. Previously, we showed that gestational and lactational exposure to an environmentally relevant mixture of BFRs disrupts proteins of the adherens junctions in rat dam mammary glands at weaning. Here, we hypothesize that perinatal exposure to the same BFR mixture also disrupts junctional proteins and signaling pathways controlling mammary gland development in pups. Dams were exposed through diet to a BFR mixture based on the substances in house dust; doses of the mixture used were 0, 0.06, 20, or 60 mg/kg/day. Dams were exposed continuously beginning prior to mating until pups’ weaning; female offspring were euthanized on postnatal day (PND) 21, 46, and 208. The lowest dose of BFRs significantly downregulated adherens junction proteins, E-cadherin, and β-catenin, and the gap junction protein p-Cx43, as well as thyroid hormone receptor alpha 1 protein at PND 46. No effects were observed on estrogen or progesterone receptors. The low dose also resulted in a decrease in cleaved caspase-3, a downward trend in PARP levels, proteins involved in apoptosis, and an upward trend in proliferating cell nuclear antigen, a marker of proliferation. No effects were observed on ductal elongation or on the numbers of terminal end buds. Together, our results indicate that gestational and lactational exposure to an environmentally relevant mixture of BFRs disrupts cell-cell interactions, thyroid hormone homeostasis and the proliferation-apoptosis balance at PND 46, a critical stage for mammary gland development.
Brominated flame retardants (BFRs) are chemicals widely used in consumer products to reduce flame propagation and ignition rates (Aasen et al., 2016; Birnbaum and Staskal, 2004). They can be found in many items containing synthetic polymers, including electronics, vehicles, and home furnishings. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCDD) have been extensively used as BFRs in North America (Janssen, 2005; Stapleton et al., 2008). Because most of the BFRs are noncovalently bound to the polymers, they leach into the environment, resulting in a widespread contamination of homes and other indoor spaces (Allen et al., 2008; Frederiksen et al., 2009). During the last decade, numerous studies have raised concerns about their persistence, bioaccumulation, and potential toxicity in animals and humans (Birnbaum and Staskal, 2004; Sjodin et al., 2003). Several studies have also highlighted the potential of various congeners of BFRs to disrupt the endocrine system. Specifically, exposure to PBDEs and HBCDD has been associated with adverse effects on thyroid and ovarian function and signaling (Ema et al., 2008; Kim et al., 2014; Lefevre et al., 2016; Lilienthal et al., 2005; Tung et al., 2016,, 2017). As a result, PBDE and HBCDD use, sale, offer for sale, and import have been restricted in Europe and North America (Environment and Climate Change Canada, 2008, 2013). However, restricted BFRs continue to be released from sources that remain in the built environment or waste streams and their environmental persistence will result in continued human exposure for years to come.
Human exposure occurs mostly through dust ingestion, food consumption, and dermal absorption (Besis and Samara, 2012; Janssen, 2005). BFRs can be detected in human serum, adipose tissue, placenta, umbilical cord, and breast milk (Birnbaum and Staskal, 2004; Hites, 2004; Mazdai et al., 2003; Shaw et al., 2010; Toms et al., 2009; Zota et al., 2013). Toddlers are the most exposed, in part due to their ingestion of dust given their closer proximity to ground and hand-to-mouth contact, in addition to gestational and lactational exposure via their mother (Hites, 2004; Mazdai et al., 2003; Toms et al., 2009; Zota et al., 2013).
Unlike most organs, mammary gland development occurs mainly after birth in a multistage process. At birth, the mammary epithelium is rudimentary (Hinck and Silberstein, 2005). Development resumes at the onset of puberty as the ovaries produce a surge of estrogens and progesterone (Mallepell et al., 2006). Thyroid hormones also play an important role in mediating ductal outgrowth (Vonderhaar and Greco, 1979). Under this hormonal surge, highly proliferative structures called terminal end buds (TEB) form at the end of the ductal trees (Paine and Lewis, 2017) and drive elongation of the epithelium during puberty (Paine and Lewis, 2017). A second phase of development occurs during pregnancy and lactation, with the differentiation of milk-secreting structures called alveoli (Hurley, 1989; Sternlicht et al., 2006). After lactation, at weaning, the gland undergoes an important phase of apoptosis and regression named involution and returns to the pregestational stage (Hurley, 1989; Masso-Welch et al., 2000). The development of the gland is tightly regulated by the interaction between multiple hormones, rendering this organ particularly vulnerable to the adverse effects of exposure to endocrine disruptors (EDs).
Modifications of the in utero environment by exposures to endocrine modifying chemicals could interfere with maturation of the endocrine signaling pathways of the offspring and cause adverse effects later in life (Diamanti-Kandarakis et al., 2009; Fenton, 2012; Fenton et al., 2012; Soto and Sonnenschein, 2010). Mammary development relies on 3 critical windows of growth and differentiation: fetal life, puberty, and pregnancy/lactation (Hassiotou and Geddes, 2013), when the glands are the most susceptible to exogenous factors such as EDs (Fenton, 2012; Fenton et al., 2012). Exposure to EDs during those periods has been associated with developmental defects and breast cancer later in life (Fenton, 2012; Fenton et al., 2012). Accordingly, female Sprague Dawley rats perinatally exposed to DE-71, a mixture of pentabrominated PBDEs, showed a significant delay in mammary gland ductal outgrowth associated with reduced elongation and altered structure at postnatal day 21 (PND 21) (Kodavanti et al., 2010). More recently, we showed that gestational and lactational exposure to an environmentally relevant mixture of BFRs resulted in reduced serum levels of T4 and altered adherens junctions in the mammary glands of dams at weaning (Dianati et al., 2017; Tung et al., 2016).
Adherens junctions are protein complexes composed of transmembrane cadherins and cytoplasmic catenins (Lien et al., 2006). They are involved in the physical association between cells, cell polarity, growth, structure, migration, and differentiation, as well as in signaling (Incassati et al., 2010). We showed that exposure to BFRs decreased the protein levels of one of the active phosphorylated forms of β-catenin (p-β-cateninSer675) and its interaction with E-cadherin, likely in a Protein kinase A (PKA)-dependent manner (Dianati and Plante, 2017; Dianati et al., 2017). Interestingly, these effects were observed only at the lowest, environmentally relevant dose of the mixture. β-catenin plays important roles in mammary development through its pleiotropic functions in cell adhesion, signal transduction, and regulation of gene expression (Incassati et al., 2010). An appropriate β-catenin signal is required for normal development of the mammary gland (Tepera et al., 2003), and its dysregulation has been associated with developmental defects and carcinogenesis (Incassati et al., 2010). Similarly, loss of E-cadherin and β-catenin interaction has been correlated with poor clinical outcome in breast cancer (Dolled-Filhart et al., 2006). We showed that β-catenin and E-cadherin interact with connexins (Cxs), the proteins forming gap junctions (GJs), during most mammary gland developmental stages (Dianati et al., 2016). GJs are specialized transmembrane channels that allow direct communication through the cytoplasm of adjacent cells (El-Saghir et al., 2011). GJs are crucial for the proper development of the mammary gland (Stewart et al., 2015; Talhouk et al., 2005), and dysregulation of gap junctional intercommunication (GJIC) or Cxs has been associated with delayed development and impaired function of the mammary gland, as well as breast carcinogenic progression (Naus and Laird, 2010).
Expression of Cxs, catenin, and cadherin is regulated by multiple hormones (de Montgolfier et al., 2011; Grummer et al., 1999; Lye et al., 1993; Mitchell et al., 2001; Nguyen and Neville, 1998; Petrocelli and Lye, 1993; Satterfield et al., 2007; Stelwagen and Singh, 2014; Stock and Sies, 2000; Winterhager et al., 1991) and dysregulated by various EDs (Chen et al., 2015; de Freitas et al., 2016; Dianati and Plante, 2017; Dianati et al., 2017; Salian et al., 2009; Tsang et al., 2013; Wang et al., 2015). Disruption of expression of these proteins is associated with the epithelial to mesenchymal transition (EMT), a process by which epithelial cells acquire mesenchymal characteristics that enable them to migrate into surrounding tissues (Eger et al., 2000). Dysregulation of EMT is a crucial step during breast cancer progression (Hanahan and Weinberg, 2000; Kalluri and Neilson, 2003).
Because BFRs have adverse effects on the lactating mammary glands of dams, we hypothesized that the exposure of pups in early life to the same environmentally relevant BFR treatment will also have observable effects on mammary gland development and signaling pathways that are manifested later in life. Specifically, the current study reports the effects of gestational and lactational exposure to an environmentally relevant mixture of PBDEs and HBCDD on cell-cell interactions, hormone signaling, and development in the mammary glands of offspring before puberty (at PND 21), after puberty (PND 46), and in adulthood (PND 208).
MATERIALS AND METHODS
BFRs mixture formulation
Formulation of the BFRs mixture was described previously (Ernest et al., 2012). Briefly, 3 technical PBDE mixtures (DE-71, DE-79, and BDE 209) and 1 HBCDD mixture were combined to yield a ratio of PBDEs congeners and HBCDD comparable to the median levels observed in Boston house dust (Allen et al., 2008; Stapleton et al., 2008). This BFRs mixture was incorporated into an isoflavone-free diet (Teklad Global 2019 diet; Harlan Laboratories, Madison, Wisconsin) with 4.3 g/kg corn oil. Diets were formulated to contain 0, 0.75, 250, or 750 mg of BFRs mixture/kg. These diet formulations were intended to deliver nominal doses of 0, 0.06, 20, and 60 of body weight/day (mg/kg/day), respectively. The low dose was estimated to be a close approximation of maximum human exposure, based on a dust ingestion rate of 100 mg/day in children (16.5 kg body weight) and the scaling of dose from humans to rodents (1:6.9, human to rat body surface area ratio). This is the third study examining the effects of dietary exposure to this mixture. The previous studies involved subchronic exposure of sexually mature males (Ernest et al., 2012) and exposure during gestation only with assessment of fetal toxicity (Berger et al., 2014). Dietary levels were confirmed in the initial study and were published (Poon et al., 2014). Relative exposure levels have been confirmed for the animals in the current study (Tung et al., 2016), as well as in the previous 2 (Berger et al., 2014; Poon et al., 2014) by analysis of PBDE levels in various tissues.
Animals
All procedures and animal studies were conducted in accordance with the procedures and principles as provided by the Canadian Council on Animal Care and were reviewed and preapproved by the Health Canada Animal Care Committee (Protocol No. 2012-015). Animals and the treatment method were described in detail previously (Tung et al., 2016). Briefly, virgin female Sprague Dawley rats were obtained from Charles River Laboratories (Charles River, St-Constant, Quebec, Canada). Following 1 week of acclimatization to the control diet, animals were randomized to 1 of 4 BFR treatments and fed with a BFR supplemented diet for 2–4 weeks before mating (Tung et al., 2016). During this period, estrous cyclicity was evaluated by analyzing vaginal cytology, as previously described (Goldman et al., 2007). Females in proestrus were caged with proven breeder male Sprague Dawley rats (maintained on the control diet) overnight. After mating, females were returned to their assigned cages and provided with water and the appropriate dietary mixture ad libitum, throughout gestation and lactation (between 9 and 14 dams for all groups). All dams were allowed to deliver (PND 0). Dams and pups body weights were recorded at intervals throughout the treatment period. Litter size was balanced at 8 pups per dam at PND 4 (Dianati et al., 2017; Tung et al., 2016). One female pup per litter was euthanized by exsanguination under isoflurane anesthesia at PND 21, PND 46, and PND 208. Left abdominal and both inguinal mammary glands (pairs 4 and 5, respectively) were dissected, weighed, and immediately snap-frozen after excision then stored at −80°C. There were used for Western blot analyses. Right abdominal mammary glands were immediately transferred onto large slides then fixed in Carnoy's fixative for subsequent whole-mount studies. Upper-thoracic and thoracic mammary glands (pairs 2 and 3, respectively) were excised, embedded in Tissue-Tek O.C.T compound (VWR International, Ville Mont-Royal, Quebec, Canada) on dry ice and stored at −80°C for subsequent histology and immunofluorescence analyses.
Western blots
Abdominal (pair 4) or inguinal (pair 5) snap-frozen glands were mechanically ground into a powder on dry ice. Powdered samples once weighed were homogenized in ice-cold triple detergent lysis buffer (pH 8) (Tris 50 mM, NaCl 150 mM, 0.02% sodium azide, 0.1% SDS, 1% Nonidet P40, 0.5% sodium deoxycholate) supplemented with 1.25 M of NaF, 1 M of NaVO3, and Halt Protease and Phosphatase Cocktail Inhibitor (ThermoFisher Scientific). After sonication, samples were centrifuged at 13 000 rpm (10 min at 4°C). Supernatants were aliquoted and stored at −80°C until further processing. All steps were performed on ice. Protein concentrations were determined using the Pierce BCA protein assay kit (ThermoScientific, Rockford, Illinois). For each sample, total protein was separated using SDS-PAGE gels (TGX Stain-Free FastCast Acrylamide kit, 10%, Bio-Rad, Mississauga, Ontario, Canada) and transferred onto PVDF membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). After transfer, total lane proteins were visualized using the ChemiDoc MP imaging system (Bio-Rad). Membranes were blocked with TBS-Tween 0.1% supplemented with 3% bovine serum albumin (BSA) or 5% dry milk and incubated overnight at 4°C with primary antibodies (Supplementary Table 1) diluted in 5% dry milk or 3% BSA in TBS-Tween 0.1%, depending on manufacturers’ recommendations. Blotted membranes were washed with TBS-Tween 0.1% 3 times for 5 min then probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Supplementary Table 1). The chemiluminescent signals were revealed using Clarity Western ECL Blotting Substrate (Bio-Rad) and visualized using the ChemiDoc MP imaging system (Bio-Rad). The density of each protein band was quantified and normalized to the total proteins in the lane using ImageLab 6.0 software (Bio-Rad).
Whole mounts and carmine stain of mammary glands
After sacrifice, right abdominal mammary fat pads were excised and processed for whole mount staining, as described with slight modifications (Plante et al., 2011). Briefly, the glands were first fixed for a minimum of 2 days in Carnoy’s solution (100% EtOH, chloroform, glacial acetic acid; 6:3:1) at room temperature. The fixed glands were washed in 70% EtOH for 1 h and then rehydrated in water for 30 min. Then, the mammary glands were stained in carmine alum stain (2% carmine and 5% aluminum potassium sulfate in water) for a minimum of 2 days. Gradually the glands were dehydrated through an EtOH series, cleared in xylene (minimum 2 days) then mounted using Permount (ThermoFisher Scientific, Burlington, Ontario, Canada). High resolution images of mammary whole mounts were collected using a Zeiss SteREO DiscoveryV20 microscope. Whole mounts of mammary glands were analyzed with Image J software. Mammary epithelium surface area was measured by tracing the best-adjusted contour of the mammary epithelium. Epithelial elongation was measured from the lowest lymph node to the end of the longest branch of the epithelium.
Immunofluorescence and image acquisition
Immunofluorescence staining and analyses methods were described in previous publications (Dianati and Plante, 2017; Dianati et al., 2016,, 2017). Briefly, tissue cryosections (7 µm) were fixed in formaldehyde (4%) and blocked in 3% BSA dissolved in TBS-Tween 0.1%. Sections were incubated with the primary antibody (Supplementary Table 2) diluted in TBS-Tween 0.1% for 60 min at room temperature or overnight at 4°C followed with the appropriate secondary antibody (Supplementary Table 2) for 60 min at room temperature. Sections were then washed 3 times, for 5 min each, stained with 4′, 6-diamidino-2-phenylindole (DAPI), and slides were mounted with Fluoromount-G (Cedarlane, Burlington, Ontario, Canada). Immunofluorescence images were obtained with a Nikon A1R+ confocal microscopic laser equipped with a spectral detector. All the analyses were performed using NIS-elements software (version 4). The numbers of Cx43 junctional plaques were quantified using the NIS-elements software localization tool and normalized to that of the nucleus (DAPI) for each image.
Statistical analysis
All the data were analyzed using GraphPad Prism, version 6.01. First, the normal distribution of each data set and the presence of outliers were assessed with D'Agostino & Pearson omnibus normality test and ROUT' test (ESD method), respectively. Data that satisfied assumptions of normality and homoscedasticity were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison tests. Otherwise, a Kruskal-Wallis test followed by a Dunn’s multiple comparison test was used. For all experiments and statistical analyses, 1 pup per litter was evaluated.
RESULTS
Gestational and Lactational Exposure to BFRs Disrupts Adherens Junctions Proteins Without Affecting p-β-catSer675 at PND 46
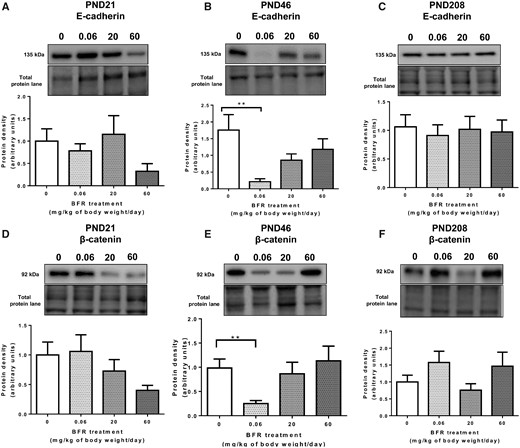
We previously reported that a low dose of the BFR mixture (0.06 mg/kg/day) reduced E-cadherin interaction with β-catenin in the adherens junctions of the mammary gland of dams exposed during pregnancy and lactation (Dianati et al., 2017). Thus, our first objective here was to assess whether gestational and lactational exposure to these BFRs had the same effects in female offspring. We quantified E-cadherin and β-catenin protein levels by Western blots at PND 21, PND 46, and PND 208 (Figure 1). Although the BFR treatment had no effects at PND 21 or PND 208 (Figs. 1A, 1C, 1D, and 1F), exposure to the 0.06 mg/kg/day dose led to a significant decrease of E-cadherin and β-catenin protein levels at PND 46 (Figs. 1B and 1E). Similar to the results in dams, no significant effects were observed for the two higher doses.
Effects of exposure to a brominated flame retardant (BFR) mixture on adherens junction protein levels in the mammary glands of the offspring. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation with a diet formulated to deliver daily nominal BFR mixture doses of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show E-cadherin (A–C) and β-catenin (D–F) protein expression at postnatal day (PND) 21 (A, D), PND 46 (B, E), and PND 208 (C, F). Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level. p-values were calculated with a Kruskal-Wallis statistical test or ANOVA. **p ≤ .01.
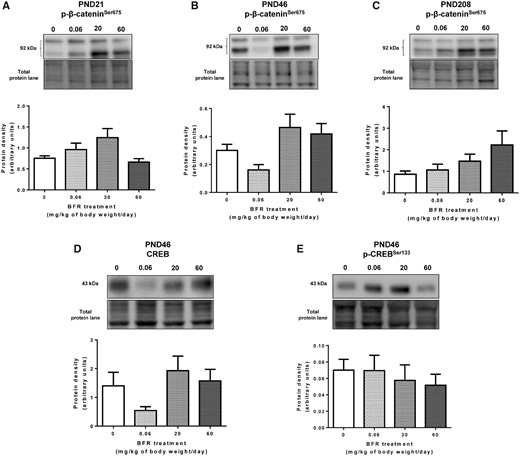
In dams treated with the BFRs, in addition to reduced E-cadherin levels, a significant decrease in p-β-catSer675 protein levels was observed in the mammary glands, as well as lower levels of p-CREB, suggesting dysregulation of the PKA pathway (Dianati et al., 2017). Again, gestational and lactational exposure did not affect the levels of p-β-catSer675 at PND 21 and PND 208 (Figs. 2A and 2C). However, at PND46, a downward trend was observed in the animals treated with the 0.06 mg/kg/day BFR dose, although it was not significant (p = .16) (Figure 2B). Similarly, although a downward trend was observed in CREB levels in the animals exposed to the 0.06 mg/kg/day dose, it did not reach statistical significance (p = .48), and no changes were observed in p-CREB levels (Figs. 2D and 2E). Together, these results suggested that gestational and lactational exposure to the lowest environmentally relevant dose of the BFR mixture results in decreased E-cadherin and β-catenin levels at PND 46 and that the PKA pathway may not be involved.
Effects of exposure to a brominated flame retardant (BFR) mixture on β-catenin signaling in the mammary glands of the offspring at postnatal day (PND) 46. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation to a diet formulated to deliver a daily nominal BFR mixture dose of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show p-β-catSer675 (A–C), CREB (D), and p-CREBSer133 (E) protein levels at PND 46. Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level. p-values were calculated with a Kruskal-Wallis statistical test or ANOVA.
BFR Treatment Dysregulates p-Cx43 Protein Levels During Postnatal Development
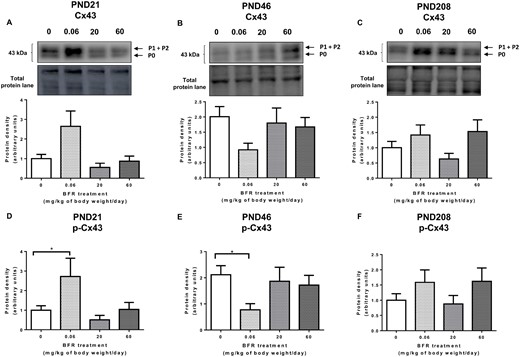
Adherens and GJs form a dynamic junctional nexus during mammary gland development (Dianati et al., 2016; Talhouk et al., 2008). We have shown that β-catenin and E-cadherin interact with Cx43 across mammary development (Dianati et al., 2016). Thus, we assessed the effects of BFRs on Cx43 protein levels in the mammary glands of the offspring. Western blot analysis showed no significant impact of the treatments on total Cx43 protein levels at all stages (Figs. 3A–C). However, exposure to the lowest dose of BFRs, 0.06 mg/kg/day, induced a significant increase in the highly phosphorylated form of Cx43 (P1–P2) at PND 21 (Figure 3D) but a significant decrease of this form at PND 46 (Figure 3E).
Effects of exposure to a brominated flame retardant (BFR) mixture on gap junction protein levels in the mammary glands of the offspring. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation to a diet formulated to deliver a daily nominal BFR mixture dose of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show Cx43 (A–C) and p-Cx43 (D–F) protein expression at postnatal day (PND) 21 (A, D), PND 46 (B, E), and PND 208 (C, F). Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level. p-values were calculated with a Kruskal-Wallis statistical test or ANOVA. *p ≤ .05.
Exposure to BFRs Does Not Affect Adherens or GJ Protein Localization at PND 46
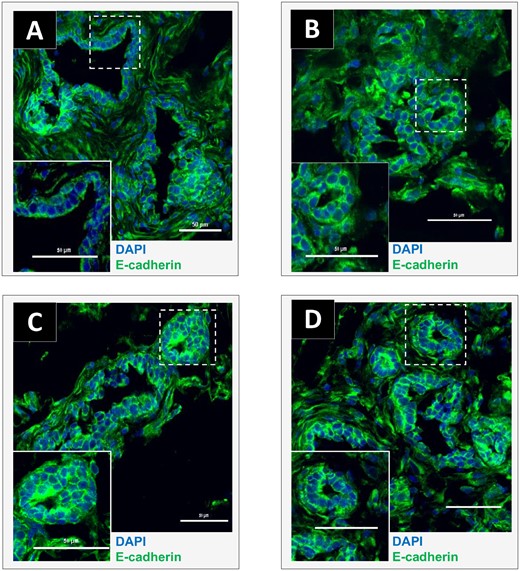
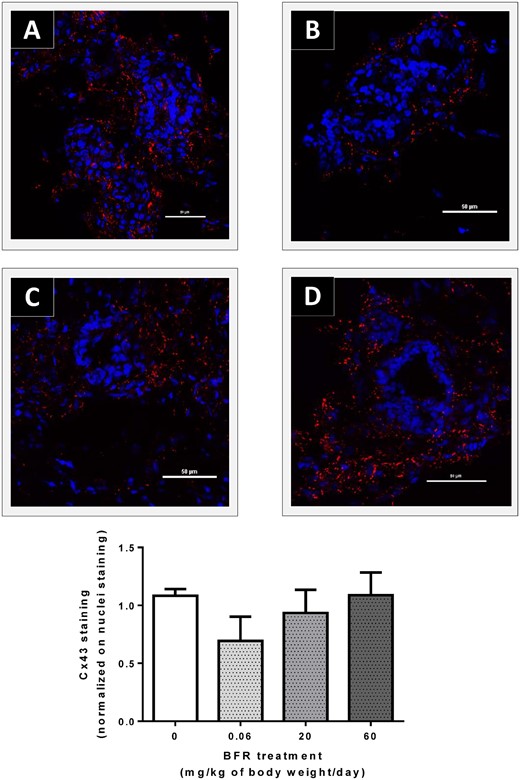
Because E-cadherin and β-catenin were downregulated by BFR treatment at PND 46, we determined whether their cellular localization was impacted using confocal immunofluorescence. No apparent changes were observed in staining intensity of E-cadherin or β-catenin among the control and treated groups (Figs. 4 and 5). p-β-catSer675 was localized at the membrane and throughout the cytoplasm and colocalized with β-catenin (Figure 5). However, no differences were observed within the treatments. Finally, we counted Cx43 junctional plaques, as p-Cx43 protein levels were reduced in the animals treated with the 0.06 mg/kg/day BFR dose. The numerical reduction in Cx43 junctional plaques in the group treated with the low 0.06 mg/kg/day dose failed to reach statistical significance (p = .37 Figure 6).
Effects of exposure to a brominated flame retardant (BFR) mixture on E-cadherin localization in the mammary glands of offspring at postnatal day (PND) 46. Cryosections were cut (7 µm) and processed for immunofluorescence staining from vehicle controls (A) or animals from the 0.06 (B), 20 (C), or 60 (D) mg/kg/day BFR mixture treatment groups. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). E-cadherin (green) was localized at the membrane and no apparent changes were noted in its protein levels or localization among groups. Images were obtained with a Nikon A1R+ equipped with a spectral detector and analyzed using NIS-elements software. (Color version of this figure is available at Toxicological Sciences online.)
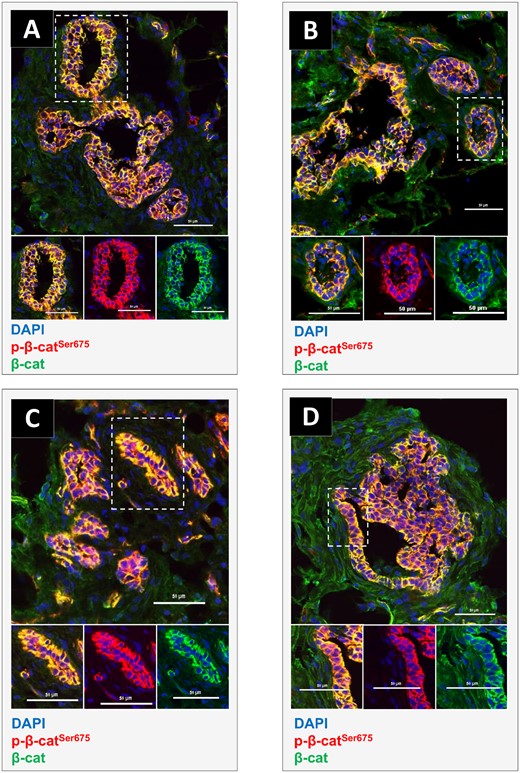
Effects of exposure to a brominated flame retardant (BFR) mixture on endogenous β-catenin and p-β-catSer675 localization in the mammary glands of offspring at postnatal day (PND) 46. Cryosections were cut (7 µm) and processed for immunofluorescence staining from vehicle controls (A) or animals from the 0.06 (B), 20 (C), or 60 (D) mg/kg/day BFR treatment groups. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). β-catenin (green) and p-β-catSer675 (red) colocalized at the membrane although no apparent change was noticed on their protein levels or localization among groups. Images were obtained with a Nikon A1R+ equipped with a spectral detector and analyzed using NIS-elements software.
Effects of exposure to a brominated flame retardant (BFR) mixture on Cx43 localization in the mammary glands of offspring at postnatal day (PND) 46. Cryosections were cut (7 µm) and processed for immunofluorescence staining from vehicle controls (A) and animals from the 0.06 (B), 20 (C) or 60 (D) mg/kg/day BFR mixture treatment groups. Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). Cx43 junctional plaques (red) were localized at the membrane; the number of plaques tended to decrease in animals treated with the 0.06 mg/kg/day dose compared to control animals. Histograms represent the means ± SEM (n = 4 pups from 4 different litters) of the number of junctional plaques normalized on the number of nuclei. Images were obtained with a Nikon A1R+ equipped with a spectral detector and analyzed using NIS-elements software.
BFR Treatment Has No Effect on EMT Markers
Downregulation of E-cadherin and junctional proteins is one of the hallmarks of EMT and contributes to breast cancer progression. Because previous reports suggested that perinatal exposure to EDs is linked to breast cancer in adulthood (Fenton, 2012; Fenton et al., 2012), we aimed to determine whether BFRs could promote EMT in rat mammary glands at PND 46. We thus determined the protein levels at PND 46 of N-cadherin and vimentin, two mesenchymal markers that are upregulated during EMT. Our Western blot analyses showed that neither mesenchymal marker was impacted in the treated groups compared to the control group (Supplementary Figs. 1A and 1B).
Together, these results suggested that the impact of gestational and lactational exposure to BFRs is maximally manifested on PND 46 during the pubertal stage of mammary gland development. Because this stage is characterized by complex hormonal changes and because BFRs can act as EDs, we then examined whether the levels of hormonal receptors were affected by the treatments at PND 46.
BFR Treatment Downregulates Thyroid Hormone Receptor Alpha1 Protein Levels, Whereas Estrogen and Progesterone Receptors Are Not Affected at PND 46
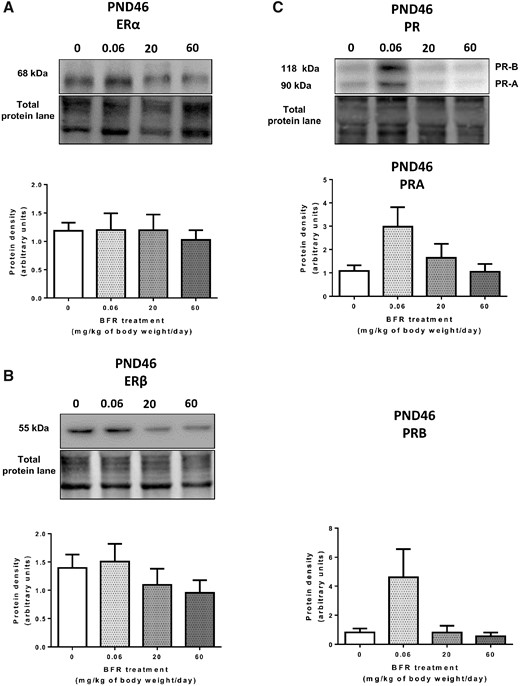
Estrogens and progesterone act via their receptors ERα and PR-B to drive mammary elongation and side branching, respectively (Brisken, 2008; Brisken and Ataca, 2015). Western blot analysis showed no effects on ERα and ERβ protein levels (Figs. 7A and 7B). A numerical increase in PR-A and PR-B levels was seen in glands from 0.06 mg/kg/day exposed PND 46 females, but these results did not reach statistical significance (p = .46 [PR-A] and p = .08 [PR-B]; Figure 7C).
Effects of exposure to a brominated flame retardant (BFR) mixture on estrogen and progesterone receptors protein levels in the mammary glands of offspring. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation to a dietary BFR mixture formulated to deliver daily nominal mixture doses of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show ERα (A), ERβ (B), and PR-A/B (C) protein expression at PND 46. Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level.
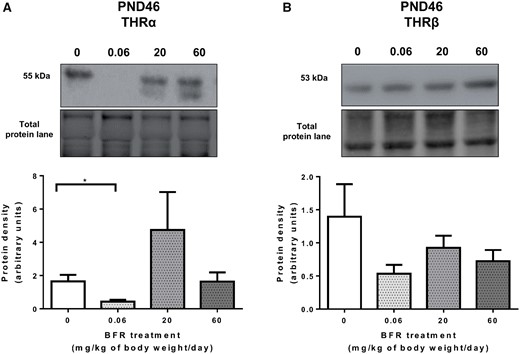
Thyroid hormones also play an important role in the development and function of mammary glands (Conde et al., 2006; Neville et al., 2002; Vonderhaar and Greco, 1979). This BFR treatment significantly reduced serum T4 in dams exposed to the highest dose of the BFR mixture, as well as in female pups perinatally exposed to the two highest doses at PND 21 (Tung et al., 2016). Therefore, we analyzed whether mammary glands thyroid hormone receptors α1 (THRα1) and β (THRβ) were affected in PND 46 females (Figure 8). Levels of THRβ protein remained unaffected (Figure 8B), while corresponding THRα1 levels were significantly downregulated only in the animals treated with the 0.06 mg/kg/day dose of BFRs compared to the control group (Figure 8A).
Effects of exposure to a brominated flame retardant (BFR) mixture on the protein levels of thyroid hormone receptors in the mammary glands of offspring. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation to a dietary BFR mixture formulated to deliver a daily nominal dose of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show thyroid hormone receptor (THR)α1 (A) and TRHβ (B) protein expression at postnatal day (PND) 46. Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level. p-values were calculated with a Kruskal-Wallis statistical test or ANOVA. *p ≤ .05.
BFR Treatment Disrupts the Proliferation-Apoptosis Balance at PND 46
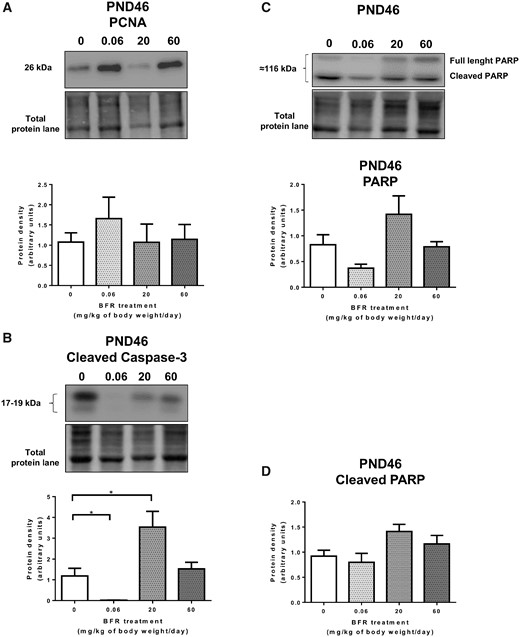
Puberty is a period of dynamic growth, tissue organization, and apoptosis during ductal development of the body cells of the TEBs, leading to the cavitation of ducts (Paine and Lewis, 2017). At age PND 46, there is a continued postpubertal mammary gland growth with terminal ends beginning to differentiate at the edges of the fat pad (Filgo et al., 2016). We thus assessed proliferation and apoptosis by analyzing proliferating cell nuclear antigen (PCNA), PARP, cleaved-PARP, and cleaved caspase-3 protein levels. A nonsignificant increase (p = .057) in PCNA protein levels in the animals treated with the 0.06 mg/kg/day dose was observed (Figure 9A). In addition, Western blot results showed no effects on the cleaved-PARP protein levels (Figure 9D), although PARP levels tended to decrease (p = .16) (Figure 9C). However, a significant decrease was observed in the cleaved caspase-3 protein levels in the animals treated with the low BFR dose compared to the control group, whereas an increase was observed at the medium dose (Figure 9B). These results suggest that the low dose of BFRs dysregulates the proliferation-apoptosis balance in the PND 46 female mammary gland.
Effects of exposure to a brominated flame retardant (BFR) mixture on protein levels of proliferation and apoptosis markers in the mammary glands of offspring. Quantitative Western blot of total proteins extracted from the mammary glands of control pups or pups exposed during gestation and lactation to a dietary BFR mixture formulated to deliver a daily nominal dose of 0.06, 20, or 60 mg/kg of body weight/day (mg/kg/day). Graphs show proliferating cell nuclear antigen (PCNA) (A); cleaved caspase-3 (B), PARP (C), and Cleaved-PARP (D) protein levels at PND 46. Histograms represent the means ± SEM (n = 9–14 pups, 1 per litter) for each band normalized to the total protein level. p-values were calculated with a Kruskal-Wallis statistical test or ANOVA. * p ≤ .05.
Exposure to BFRs Does Not Affect Mammary Gland Morphogenesis at PND 46
Finally, we assessed whether the observed effects of the low dose of BFRs on junctional proteins, hormone signaling, proliferation, and apoptosis affected global morphogenesis of the mammary gland at puberty. Consistent with normal development of the mammary gland at PND 46, TEBs were numerous, ducts were reaching toward the periphery of the fat pad and side branches were abundant in whole mount analysis (Supplementary Figs. 2A and 2B). No effects of the BFRs were observed on epithelial elongation (Supplementary Figure 2A), epithelial surface area (Supplementary Figure 2B) or the number of TEBs (Supplementary Figure 2C) in treated animals. In addition, exposure to BFRs had no effects on either body weight or on mammary gland weight (Supplementary Figs. 2D–F). Together, these data suggested that although the treatment disrupted junctional proteins, THRα1, and proliferation-apoptosis homeostasis, gestational and lactational exposure to an environmentally relevant mixture of BFRs did not impact global mammary gland development of the female offspring at PND 46.
DISCUSSION
Adherens Junctions Are Sensitive Targets of BFR Exposure
Exposure to the lowest dose of the BFR mixture dysregulated proteins from the adherens junctions in both this study and our previous study. In dams exposed during pregnancy and lactation, we showed decreases in the expression of p-β-catSer675 and its interaction with E-cadherin at the cell membrane (Dianati et al., 2017). Although the levels of β-catenin were also decreased, the effect was not statistically significant. In female pups from these same dams, the mammary glands collected at PND 46 showed levels of E-cadherin and β-catenin that were significantly decreased. These results suggest that adherens junctions are especially sensitive to the effects of BFRs. Many studies have demonstrated a decrease in adherens junction protein levels upon exposure to pollutants. For example, E-cadherin downregulation was also detected in human mammary stem cells (Yang et al., 2013), in rat dorsolateral prostate (Huang et al., 2018), and colon cancer cells (Chen et al., 2015) when exposed to bisphenol A (BPA), another endocrine-disrupting chemical that has been studied extensively. In addition, our previous results showed a similar later downregulation of E-cadherin and of hepatic connexins 45 days after the last exposure to hexachlorobenzene in female rat livers (Plante et al., 2002,, 2005).
Together, these findings demonstrated that the BFR treatment disrupted adherens junctions both in the dams’ lactating glands and in the mammary glands of the pups exposed in utero and throughout lactation. Interestingly, the decrease in adherens junction protein levels seems to involve dysregulation of PKA signaling in dams (Dianati et al., 2017) but not in their offspring at PND 46 because p-β-catSer675, CREB, and p-CREB levels were not affected by exposure to BFRs. These findings support the hypothesis that exposure to EDs, such as BFRs, can have different effects based on the window of exposure but that adherens junctions seem to be a prime target (Diamanti-Kandarakis et al., 2009).
Adherens junctions play a major role in mammary gland development. As a result, disruption of E-cadherin in the normal mammary gland has been associated with impaired epithelial survival and function of the gland (Boussadia et al., 2002; Delmas et al., 1999), and to disorganization of the luminal layer of the mammary gland in mice (Daniel et al., 1995). E-cadherin interacts with β-catenin to form a cell-cell adhesion complex (Andrews et al., 2012). In addition, β-catenin acts as a transcription factor that mediates the canonical WNT/β-catenin signaling pathway (Incassati et al., 2010). The E-cadherin/β-catenin membrane complex prevents β-catenin translocation to the nucleus, where it induces the expression of genes that promote cell proliferation and survival (Incassati et al., 2010). Dysregulation of β-catenin signaling led to impaired lobuloalveolar development and has been significantly correlated with poor clinical outcome in breast cancer (Dolled-Filhart et al., 2006; Tepera et al., 2003). Moreover, loss of β-catenin from the cell-cell adhesive complex and its cytoplasmic accumulation and nucleus translocation occur during breast cancer progression (Incassati et al., 2010; Teuliere et al., 2005). Despite our observations of molecular changes in adherens junctional proteins, we did not observe any morphological effects on mammary gland development in either dams or pups. Whether these changes have long-term effects on breast cancer promotion remains to be determined.
Similar to what has been observed in dams, p-β-catSer675 was localized not only to the cell membrane but also throughout the cytoplasm at PND 46. Phosphorylation of β-catenin at Ser675 was shown to prevent its degradation and promote its nuclear transcriptional activity (Incassati et al., 2010; Spirli et al., 2013; Taurin et al., 2006). To date, only a few articles have studied the role and/or expression of p-β-catSer675; most of these are in vitro studies (Estus et al., 2016; Fanti et al., 2014; Hino et al., 2005; Zheng et al., 2018; Zhu et al., 2012). To our knowledge, our studies are the first to determine the localization of p-β-catSer675 in mammary gland tissues. Additional studies are thus required to fully understand the role of this phosphorylation site in vivo and its impact on mammary gland development and function.
T4 Levels and THRα1 Are Decreased Upon Exposure to BFRs During the Gestation-Lactation Period in Dams and in Offspring at PND 46, Respectively
Many studies have demonstrated an effect of BFRs on thyroid hormone homeostasis, including decreases in T4 levels and increases in thyroid stimulating hormone levels, in both exposed pregnant rats and their female offspring (Ernest et al., 2012; Kim et al., 2009; Kodavanti et al., 2010; Tung et al., 2016; van der Ven et al., 2006). However, to the best of our knowledge, the effects of BFRs on THR protein expression in the mammary gland have not been assessed before this. Previous reports suggested that decreased serum T4 levels could be linked to a competition between some BFRs and T4 for binding to human transthyretin, the T4-transporting protein in plasma, as demonstrated in vitro (Hamers et al., 2006; Meerts et al., 2000). Alternatively, exposure to PBDEs can increase expression of enzymes that metabolize T4, leading to increased removal from circulation and reduced serum levels (Szabo et al., 2009). Elevated activity of enzymes associated with these pathways was observed in the livers of exposed dams and pups in whom reduced T4 was observed, suggesting that BFR-enhanced hepatic metabolism of TH underlies the observed reduction in serum T4 (Tung et al., 2016). However, no change in circulating T4 was observed in animals exposed to the 0.06 mg/kg diet at any stage of development.
Our results show a significant downregulation of THRα1, but not THRβ, in PND 46 female offspring exposed in utero and during lactation to the BFR mixture but only in animals from the 0.06 mg/kg diet group. Unlike ER and PR, the expression of THRs as predictive markers of cancer has been assessed poorly (Ditsch et al., 2013; Jerzak et al., 2015; Silva et al., 2002; Smallridge and Latham, 1980), and the exact role of THRs in breast cancer remains to be elucidated. High THRα expression has been associated with disease-free survival in women with invasive breast cancer (Conde et al., 2006), although the specific role of THRα isoforms was not assessed. Other studies have shown that THRα2 expression correlates with survival (Ditsch et al., 2013), and patients who had low THRα1 and high THRα2 expression had the highest overall survival (Jerzak et al., 2015).
Thyroid hormones/THRs are involved in mammary gland development and differentiation (Hovey et al., 1999; Vonderhaar and Greco, 1979). Specifically, they have been shown to regulate tertiary epithelial branching and lobuloalveolar development in mice (Hovey et al., 2002). Alteration of thyroid hormone/THR signaling has also been associated with impaired mammary development (Hovey et al., 1999; Vonderhaar and Greco, 1979) or breast cancer (Conde et al., 2006; Ditsch et al., 2013; Jerzak et al., 2015). Whether the observed changes in thyroid signaling in dams or female pups at PND 21 and PND 46 have long-term effects on mammary development and carcinogenesis remains to be investigated. In addition, the relationship between the effects observed in dams and those observed later in pups is intriguing.
Gestational and Lactational Exposure to BFR Dysregulates p-Cx43 Protein Levels Later in Postnatal Development
Many studies have demonstrated that components of adherens junctions interact, colocalize and immunoprecipitate with components of GJ (Dianati et al., 2016; Talhouk et al., 2008, 2013; Wu et al., 2003; Xu et al., 2001). Moreover, E-cadherin and Cxs are downregulated concomitantly in many tumors and can be regulated through common pathways (Plante et al., 2005, 2006). Because BFR exposure disrupted adherens junction proteins, we investigated whether Cx43, the only Cx that is expressed at significant levels at PND 21, PND 46, and PND 208, was impacted (Dianati et al., 2016; McLachlan et al., 2007). Cx43 GJs are essential for mammary gland development and function (Plante and Laird, 2008). An autosomal dominant mutant of Gja1, the gene encoding Cx43, led to low levels of Cx43, specifically P1–P2 species, delayed mammary development at puberty and ultimately impaired milk ejection at parturition (Plante and Laird, 2008). Although Cx43 has been considered a tumor suppressor for a long time, its role in breast cancer is still controversial (Busby et al., 2018; McLachlan et al., 2007). We observed no effects of the BFRs on total Cx43 protein levels. However, the highly phosphorylated form of Cx43, widely called P1–P2, was significantly upregulated at PND 21. Current investigations are ongoing to further elucidate the consequences of p-Cx43 upregulation at PND 21. In contrast, consistent with the observed decrease in adherens junction proteins, P1–P2 Cx43 species were downregulated at PND 46. Cx43 phosphorylation is typically associated with its localization at the cell membrane and with functional GIJC (Laird, 2006; Lampe and Lau, 2004). P1–P2 were shown to be typically enriched in GJ plaques, whereas the Cx43-P0 species are generally found in intracellular compartments (Laird, 2006). Thus, we examined whether membrane localization was also impacted at PND 46. We observed a numerical reduction in Cx43 gap junctional plaques in the low 0.06 mg/kg/day diet, but this decrease was not statistically significant. Interestingly, a decrease in Cx43 was observed both at the mRNA and protein levels in testes of males exposed to BDE-209 during lactation at PND 21 and PND 28 (Sarkar and Singh, 2017). Similarly, another BFR—tetrabromobisphenol A—was shown to inhibit GJIC in an epithelial liver cell line (Samuelsen, 2014). The long-term consequences and the mechanisms involved in p-Cx43 dysregulation by BFRs in mammary glands remain to be determined.
Interconnection Between the Effects of BFR Treatment on Cell-Cell Interactions and Thyroid Homeostasis
Exposure to BFRs resulted in decreased levels of the adherens junction proteins E-cadherin and β-catenin, as well as the GJ protein p-Cx43, concomitant with a significant decrease in THRα1 protein expression at PND 46. These results suggest a relationship between THRα1 signaling and junctional protein regulation in the mammary gland. A few studies have investigated the regulation of junctional proteins in other tissues. For instance, THRα1 was shown to stimulate the transcription of the Ctnnb1 gene, the gene encoding β-catenin, in precursor cells of the mouse intestinal epithelium through its binding to an intronic TRE element, and hypothyroidism led to downregulation of Ctnnb1 expression and translation (Plateroti et al., 2006). T3 also increased the stabilization and transcriptional activity of β-catenin in rodent liver cells, thus promoting proliferation (Fanti et al., 2014). Interestingly, in the same study, T3 treatment increased β-catenin phosphorylation at Ser675; administration of a PKA inhibitor in vivo and in vitro inhibited p-β-catSer675 and simultaneously decreased Cyclin-D1 expression, blocking hepatocyte proliferation (Fanti et al., 2014). T3 was reported to regulate E-cadherin and β-catenin expression in the stomach during the metamorphosis of the toad Rhinella arenarum (Izaguirre and Casco, 2010). Similarly, links between Cx43 expression and thyroid hormones have been reported. T3 directly stimulated Cx43 expression in brook trout Sertoli cells (de Montgolfier et al., 2011). In addition, Cx43 knockdown was associated with a significant increase in β-catenin transcriptional activity and enhanced human neuronal differentiation (Rinaldi et al., 2014). However, to the best of our knowledge, no study has examined the interconnection between thyroid hormones/THR signaling and β-catenin, E-cadherin, and Cx43 expression in the mammary gland. Further in vivo and in vitro investigations will help elucidate these relationships.
BFR Treatment Induces Dysregulation of the Balance Between Apoptosis and Proliferation but Has No Effect on Global Mammary Gland Development
Most of the effects of our BFR treatment occurred at PND 46, a period of continued cell proliferation and apoptosis for ductal elongation and side branching (Humphreys et al., 1996; Masso-Welch et al., 2000; Paine and Lewis, 2017; Williams and Daniel, 1983). We thus examined the impact of BFR exposure on apoptosis and proliferation using cleaved caspase-3 and PARP and PCNA levels. At the low dose of BFRs, the levels of the two markers of apoptosis decreased, whereas the proliferation marker tended to increase, suggesting a dysregulation of the proliferation-apoptosis balance. Interestingly, independent studies have demonstrated that in MCF-7 breast cells, concomitant exposure to DE-71 and 17β-estradiol reduced apoptosis (Kwiecińska et al., 2011), whereas some PBDEs stimulated proliferation (Li et al., 2012; Mercado-Feliciano and Bigsby, 2008), thus supporting our results. Inhibition of caspase-3 activity has been shown to impair TEB cavitation in mouse mammary glands at puberty (Sreekumar et al., 2017).
Given the disruption of cell-cell interactions, thyroid hormone homeostasis and proliferation-apoptosis balance, we sought to determine the effects of our BFR treatment on mammary gland development. Our results suggest that global epithelial growth was not affected by exposure to the BFR mixture, and no differences were observed in mammary weight, epithelial elongation and surface, or TEB number at PND 46. In contrast, a previous study showed that perinatal exposure to a commercial PBDE mixture, DE-71, induced a significant delay in mammary gland development in female offspring, as demonstrated by an inhibition of epithelial outgrowth at PND 21 and fewer TEBs and branches present at the time of puberty (Kodavanti et al., 2010). The discrepancy between these results and ours could be due to the many differences in study design (Kodavanti et al., 2010). Although using DE-71 alone can provide information on the direct impact of this mixture, our BFR mixture allows for a better characterization of the impact of BFRs in terms of environmental exposure. To the best of our knowledge, our study is the first to report the effects of gestational and lactational exposure to an environmentally relevant mixture of BFRs on mammary gland development and signaling pathways in offspring.
BFR Treatment Induces Low-Dose and Nonmonotonic Effects
“Low-dose effects” were defined by the scientific panel of the National Toxicology Program as effects observed at doses that are in the range of human exposure or at doses below those traditionally tested in toxicological studies (Melnick et al., 2002). In addition, although effects can also be observed at higher doses, they can differ from those observed at the low dose, or follow a nonmonotonic response-curve (Vandenberg et al., 2012). Nonmonotonic dose-response curves and low-dose effects are typically associated with EDs and have been observed in several in vitro, in vivo and epidemiological studies for PBDEs, diethylstilbestrol (DES), BPA, di(2-ethylhexyl) phthalates (DEHP), polychlorinated biphenyls (PCB), dioxins and dichlorodiphenyltrichloroethane (DDT), among others (Ahn et al., 2005; Alm et al., 2010; Chen et al., 2018; Fan et al., 1996; Jones et al., 2011; Lilienthal et al., 2006; Lim et al., 2008; Lind and Lind, 2011; Love et al., 2003; Mandrup et al., 2016; Melzer et al., 2010; Palanza et al., 2001; Talsness et al., 2008; vom Saal et al., 1997). In accordance, in our studies, most of the effects of the BFR treatment have been consistently observed in the mammary gland of the dams exposed to the lowest dose, but not higher doses, (Dianati et al., 2017), as well as their pups at PND 21 and PND 46 (this manuscript). Similarly, studies from our collaborators using the same environmentally relevant mixture of BFR show significant low-dose effects or nonmonotonic response-curves in different biological processes and tissues, such as hormones plasmatic levels, bone formation, thyroid gland, and ovaries structure and maturation, as well as in neurodevelopmental-related behavior in rats directly exposed to the mixture as well as their offspring across development (Berger et al., 2014; Dianati et al., 2017; Lefevre et al., 2016; Tung et al., 2017). Several other studies using technical commercial mixture of PBDEs have also demonstrated low-dose effects and nonmonotonic response-curves in vitro and in vivo (Chen et al., 2018; Lilienthal et al., 2006; Talsness et al., 2008).
Although the mechanisms involved in the low-dose effects are not completely understood, it is now well established in the literature that similar to endogenous hormones, an important number of EDs act at low doses (Vandenberg et al., 2012). The endocrine response is based on a ligand-receptor system where small changes in the levels of hormones can modify receptor occupancy and generate pleiotropic biological answer (reviewed by Vandenberg et al., 2012). Because they disturb the endocrine system by, among others, binding to the receptors or influencing hormone transport and metabolism, EDs are thought to follow the same rules as hormones (Welshons et al., 2003, 2006). Several mechanisms have been proposed to explain low-doses and nonmonotonic responses of EDs (reviewed by Vandenberg et al., 2012). Overall, it is believed that EDs can induce cellular responses at low dose, similar to hormones, whereas at higher doses they can be cytotoxic, affect several pathways or receptors, induce receptors downregulation or desensitization or activate negative feedback loop, among others (reviewed by Vandenberg et al., 2012).
Although we did not assess mechanisms underlying the low-dose effects of the BFR mixture in the mammary gland of dams (Dianati et al., 2017) and their pups, we can speculate that dysregulation of the thyroid system homeostasis could be involved. Indeed, PBDEs are structurally similar to thyroid hormones and have been shown to influence thyroid homeostasis (Blake et al., 2011; Darnerud et al., 2001; Ernest et al., 2012; Fowles et al., 1994; Hallgren et al., 2001; Hamers et al., 2006; Meerts et al., 2000; Szabo et al., 2009; Tung et al., 2016). Importantly, our low dose of the BFR mixture was chosen based on levels found in house dust thus reflecting human environmental exposure (Allen et al., 2008; Stapleton et al., 2008), whereas the higher doses were more similar to those used in other toxicological studies (Kodavanti et al., 2010; Stoker et al., 2005). Together, our results that occur consistently at the lowest environmentally relevant dose of the BFR mixture supports the now well-documented “low-dose effect” concept. This frequent occurrence of low-dose effects and nonmonotonicity in biological phenomena following exposure to EDs, and especially in mammary gland development, highlight the importance of understanding the underlying mechanisms and strengthen the overall weight of evidence supporting inclusion of low doses in future endocrine-disrupting chemical-related studies and risk assessment.
CONCLUSION
Overall, our results showed that although no effect was observed on global mammary gland development, expression levels of the adherens junction proteins E-cadherin and β-catenin and GJ protein p-Cx43, THRα1, and cleaved caspase-3 were downregulated at PND 46 in the animals that received the lowest dose of BFRs in utero and during lactation. Modifications, such as altered hormone signaling, at the time of puberty have been shown to increase the risk of breast cancer development (Fenton et al., 2012). In this study, the timing of the observed effects may have important long-term consequences, as the pubertal period is known to be sensitive for mammary development and carcinogenesis. Further analyses will allow us to determine the mechanisms involved in the effects of BFRs on cell-cell interactions, thyroid homeostasis and the apoptosis-proliferation balance in the mammary gland, as well as the long-term consequences on mammary gland development and function
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGEMNTS
Martine Caplette and Elodie Renard are thanked for their technical assistance.
FUNDING
This study was funded by Canadian Institutes of Health Research (CIHR), Institute for Human Development, Child and Youth Health (RHF No. 100625 to B.F.H. and C.G.G.), Health Canada (M.G.) and a Natural Sciences and Engineering Research Council of Canada grant (NSERC No. 418233–2012 to I.P.).
BFH and BR are James McGill Professors.
I.P. is the recipient of a Fonds de Recherche du Québec-Santé (FRQS) and Quebec Breast Cancer Foundation career award, and a Leader Founds from the Canadian Foundation for Innovation.
R-J.G received a scholarship from the Ministère de l'enseignement supérieur et de la recherche de Côte d'Ivoire and Réseau Québécois en Reproduction - CIRD.
E.D. and M.L received a scholarship from the Fondation Universitaire Armand-Frappier.




Comments