
Abstract
Aims: The aim of this study was to evaluate the five-year clinical results of a sirolimus-eluting stent (MiStent SES) with a bioabsorbable coating designed for sustained drug delivery during and after rapid polymer dissolution.
Methods and results: The five-year results from the DESSOLVE I and II trials including major adverse cardiac events (MACE), target lesion failure (TLF), target vessel failure (TVF), and stent thrombosis (ST) at five-year follow-up are reported. In DESSOLVE I, 10.3% of patients receiving the MiStent SES (3/29) had a MACE event up to five years without TLF. In DESSOLVE II, 15.1% of patients in the MiStent group (18/119) had a five-year MACE event compared to 22.0% of patients in the Endeavor group (p=0.295). TLF was 9.2% in the MiStent group and 8.5% in the Endeavor group (p=1.00). TVF was 10.1% for MiStent versus 15.3% for Endeavor (p=0.331). Up to five-year follow-up, the MiStent SES has continued to demonstrate low rates of TLR across DESSOLVE I (0.0%) and DESSOLVE II (3.4%). No ST was reported with the MiStent up to five years in the DESSOLVE I trial. In DESSOLVE II, definite or probable ST was 0.0% with MiStent and 1.7% with Endeavor up to five years.
Conclusions: The MiStent SES demonstrated long-term safety and effectiveness with low rates of five-year MACE, TLF, and TVF across these two clinical trials. ClinicalTrials.gov Identifiers: NCT01247428 and NCT01294748.
Abbreviations
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
LLL: late lumen loss
MACE: major adverse cardiac events
MI: myocardial infarction
NIH: neointimal hyperplasia
PLGA: polylactide-co-glycolic acid
SES: sirolimus-eluting stent
ST: stent thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Permanent polymers on drug-eluting stents (DES) have been linked to late stent thrombosis (ST) and DES failure due to inciting an inflammatory response1. Bioabsorbable polymer-based DES were developed to address this limitation with results supporting their use as safe and efficacious alternatives to permanent polymer DES2,3.
The MiStent® sirolimus-eluting absorbable polymer coronary stent system (MiStent SES) (Micell Technologies, Durham, NC, USA) combines sirolimus, polylactide-co-glycolic acid (PLGA) and a cobalt-chromium stent platform. PLGA contains a crystalline form of sirolimus. The stent is free of the sirolimus/PLGA mixture within 45-60 days and PLGA is fully absorbed within 90 days. The crystalline sirolimus resides in the tissue continuously eluting the drug into the target lesion tissue for up to nine months, a distinct feature of the MiStent SES4,5.
Methods
STUDY DESIGNS
The five-year MiStent results are from the DESSOLVE I single-arm, first-in human trial, and from the DESSOLVE II 2:1 randomised trial evaluating the MiStent compared to the Endeavor® Sprint DES (Medtronic, Santa Rosa, CA, USA)6-8. These studies included subjects with symptomatic ischaemic heart disease due to de novo lesions in native coronary arteries with >50% diameter stenosis.
The DESSOLVE I trial investigated early and long-term neointimal hyperplasia (NIH) and vessel healing as well as evaluating clinical outcomes (n=30)6. In the DESSOLVE II trial, in-stent late lumen loss (LLL) at nine months was significantly less (p<0.001) for the MiStent (0.27±0.46 mm, n=123) when compared to the Endeavor (0.58±0.41 mm, n=61)7.
Patients in DESSOLVE I and II were contacted for clinical follow-up and reporting of adverse events annually post index procedure for five years. The duration of dual antiplatelet therapy (DAPT) was per the country and/or study hospital standard-of-care recommendations.
CLINICAL ENDPOINT DEFINITIONS
DESSOLVE I and II used the same clinical endpoint definitions6,7. MACE was a composite of any death, myocardial infarction (MI) (Q-wave or non-Q-wave) or clinically driven target vessel revascularisation (TVR). Target lesion failure (TLF) was defined as cardiac death, target vessel MI, or clinically driven target lesion revascularisation (TLR). Target vessel failure (TVF) was defined as cardiac death, target vessel MI, or TVR. Periprocedural MI was defined as Q-wave or non-Q-wave MI prior to hospital discharge, or CK-MB elevation >3x ULN within 48 hours post PCI, with a normal CK-MB at baseline. A clinical events committee met regularly up to five years to adjudicate pre-specified events including all deaths, MI, TVR and ST.
STUDY CONDUCT
All patients provided written informed consent in accordance with the local site’s ethics committee. Participating sites and the study organisation have been described previously6-8. SAS, version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
In DESSOLVE I, MACE was 0.0% (0/30) at 30 days, 3.4% (1/29) at two years, and 10.3% (3/29) at five years. The three events reported over five-year follow-up included two non-target vessel non-Q-wave MIs and one clinically driven target vessel revascularisation. No patients had death, TLR, TLF or ST over five years. At five-year follow-up, 17.2% of patients were on dual antiplatelet therapy (DAPT).
In DESSOLVE II, follow-up at five years was available for 95.7% of patients. The MACE rate up to five years was 15.1% (18/119) for MiStent and 22.0% (13/59) for Endeavor (p=0.295) (Figure 1). Between two and five years, 8% of patients in both groups had a MACE event (10/119 for MiStent and 5/59 for the Endeavor group). No deaths were reported within 30 days of implantation in either group. Up to five-year follow-up, death was reported in 9.2% of the MiStent patients and 10.2% of the Endeavor patients (Table 1). Rates of myocardial infarction were also similar between groups up to five-year follow-up (5.9% with MiStent and 5.1% with Endeavor; p=1.00). Clinically driven TVR was 5.0% in the MiStent group and 10.2% in the Endeavor group up to five years (p=0.216), with clinically driven TLR rates being similar between the groups (3.5% with MiStent and 3.4% with Endeavor; p=1.00) (Figure 2). Stent thrombosis (probable or definite) occurred in 0.0% of patients receiving the MiStent and 1.7% of patients receiving the Endeavor stent (p=0.331). At five-year follow-up, 37.4% of patients in the MiStent group and 42.3% of patients in the Endeavor group were on DAPT.
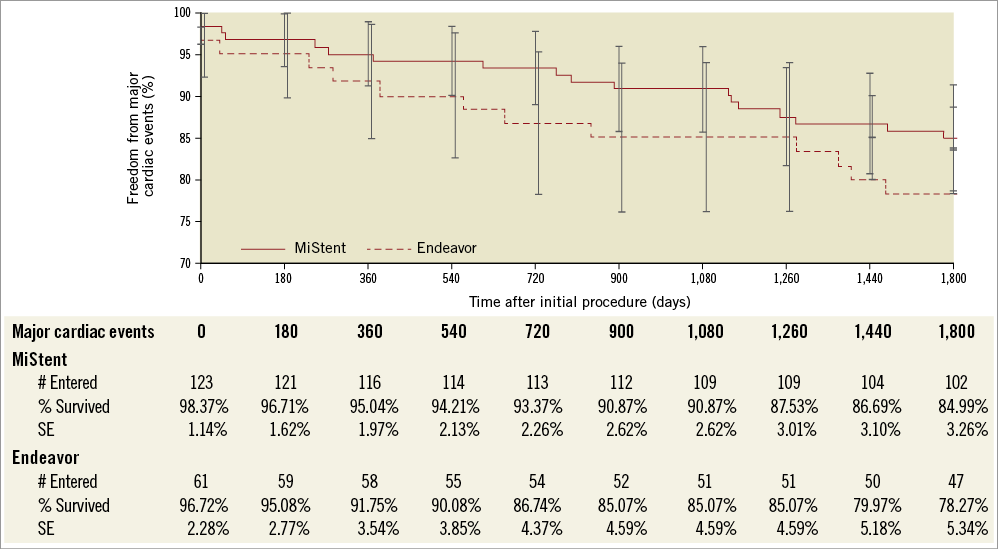
Figure 1. Survival curve - major adverse cardiac events (MACE) at five years in DESSOLVE II - intention-to-treat population.
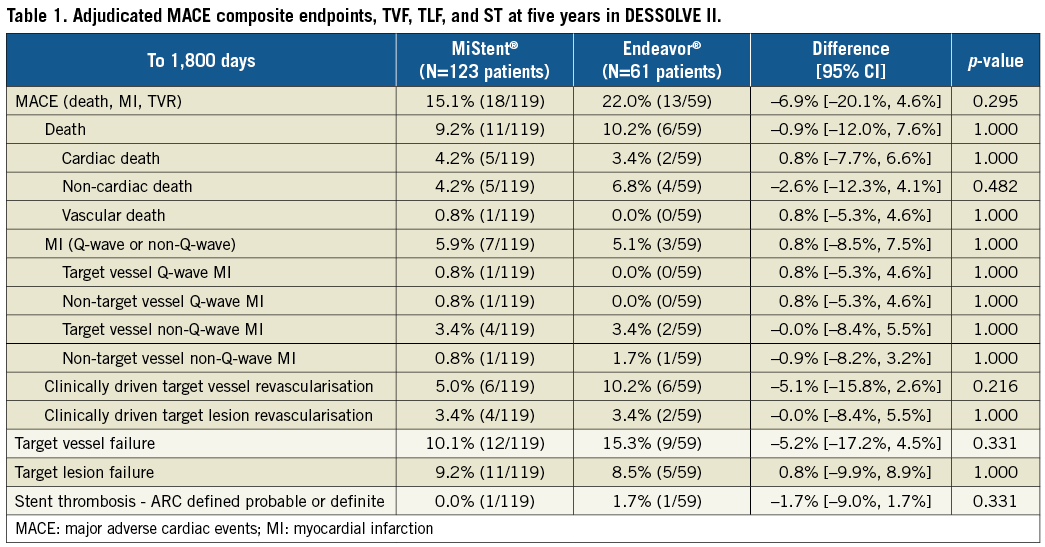
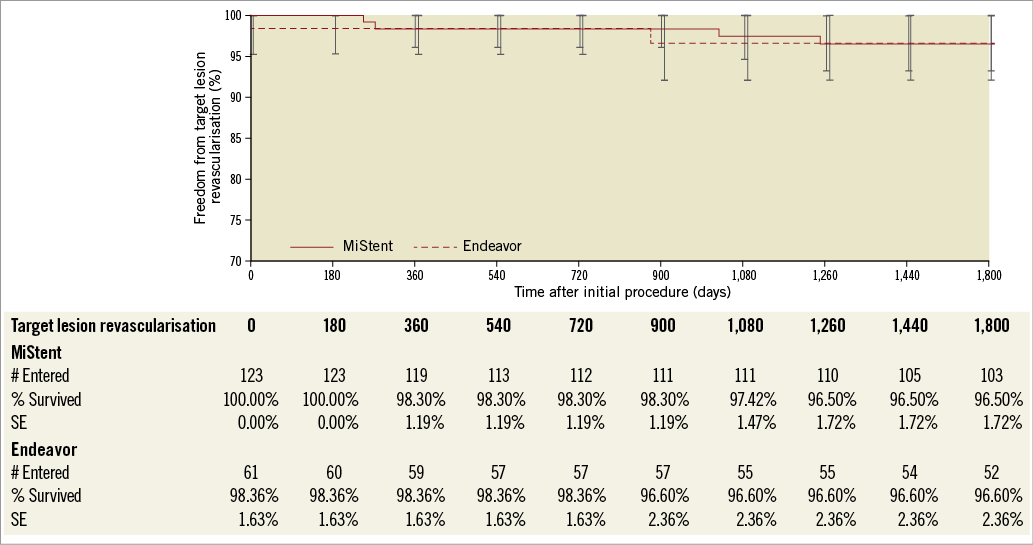
Figure 2. Survival curve - target lesion revascularisation at five years in DESSOLVE II - intention-to-treat population.
Discussion
The MiStent uses a unique crystalline sirolimus that continues to deliver the antiproliferative drug months after the polymer is absorbed, which is designed to address concerns related to late catch-up seen with some DES9-11. To address safety concerns associated with first- and second-generation DES with permanent polymers12,13, DES with fully absorbable polymer coatings such as the MiStent have been developed4.
Up to five-year follow-up, the MiStent SES has continued to demonstrate low rates of TLR across DESSOLVE I (0.0%) and DESSOLVE II (3.4%), with a combined TLR rate of 2.7%. In addition, a recent propensity-matched analysis including MiStent patients from DESSOLVE I and II and XIENCE patients from ISAR-TEST 4 demonstrated significantly lower TLR rates up to three years with MiStent vs. XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) (2.0% vs. 8.4%; p=0.04)14. These low rates of revascularisation are consistent with findings from a paired angiographic analysis from DESSOLVE I which demonstrated only 0.09 mm of NIH between early (six to eight months) and late follow-up (18 months)6,7. The DESSOLVE III randomised trial which evaluated MiStent vs. the XIENCE V stent in an all-comers population has completed enrolment and results were recently presented (https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2017/Late-breaking-trials-and-trial-updates)15.
Limitations
Similar to other DES trials, investigators could not be blinded to study device, and neither study was powered for clinical outcomes. Larger trials including more complex patients/lesions along with real-world experience will help to provide additional information on the MiStent SES.
Conclusions
Five-year follow-up data from DESSOLVE I and II show a good safety profile for the MiStent SES, with low rates of TLR. Further studies in larger populations provided additional data in 2017 (DESSOLVE III)15.
| Impact on daily practice The MiStent platform is unique in that it combines a thin-strut stent, with a rapidly bioabsorbable polymer, and crystalline sirolimus that maintains therapeutic drug levels for up to nine months. Clinical use of this device is supported by the safety profile and low rates of TLR from these two trials with five years of follow-up. |
Funding
This trial is sponsored by Micell Technologies, Durham, NC, USA.
Conflict of interest statement
W. Wijns reports receiving institutional research grants from several device companies, including Micell Technologies and Medtronic, to his previous institution, and speaker fees from Abbott Vascular, Biotronik and MicroPort, and being a co-founder of Argonauts Partners. D. Donohoe is a consultant for Micell Technologies. D. Kandzari has received consulting/educational honoraria from Boston Scientific, Medtronic Cardiovascular, and Micell Technologies, and research/grant support from Abbott Vascular, Boston Scientific, and Medtronic Cardiovascular. The other authors have no conflicts of interest to declare.




