Abstract
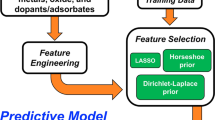
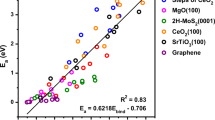
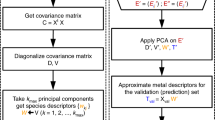
Single-atom catalysts offer high reactivity and selectivity while maximizing utilization of the expensive active metal component. However, they are susceptible to sintering, where single metal atoms agglomerate into thermodynamically stable clusters. Tuning the binding strength between single metal atoms and oxide supports is essential to prevent sintering. We apply density functional theory, together with a statistical learning approach based on least absolute shrinkage and selection operator regression, to identify property descriptors that predict interaction strengths between single metal atoms and oxide supports. Here, we show that interfacial binding is correlated with readily available physical properties of both the supported metal, such as oxophilicity measured by oxide formation energy, and the support, such as reducibility measured by oxygen vacancy formation energy. These properties can be used to empirically screen interaction strengths between metal–support pairs, thus aiding the design of single-atom catalysts that are robust against sintering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Haruta, M. Catalysis of gold nanoparticles deposited on metal oxides. CATTECH 6, 102–115 (2002).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Fiedorow, R. M. J., Chahar, B. S. & Wanke, S. E. The sintering of supported metal catalysts. J. Catal. 51, 193–202 (1978).
Bruix, A. et al. A new type of strong metal–support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts. J. Am. Chem. Soc. 134, 8968–8974 (2012).
Branda, M. M., Hernandez, N. C., Sanz, J. F. & Illas, F. Density functional theory study of the interaction of Cu, Ag, and Au atoms with the regular CeO2(111) surface. J. Phys. Chem. C 114, 1934–1941 (2010).
Flytzani-Stephanopoulos, M. & Gates, B. C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 3, 545–574 (2012).
Campbell, C. T. Catalyst–support interactions: electronic perturbations. Nat. Chem. 4, 597–598 (2012).
Campbell, C. T. & Sellers, J. R. V. Anchored metal nanoparticles: effects of support and size on their energy, sintering resistance and reactivity. Faraday Discuss. 162, 9–30 (2013).
Campbell, C. T. The energetics of supported metal nanoparticles: relationships to sintering rates and catalytic activity. Acc. Chem. Res. 46, 1712–1719 (2013).
Hemmingson, S. L. & Campbell, C. T. Trends in adhesion energies of metal nanoparticles on oxide surfaces: understanding support effects in catalysis and nanotechnology. ACS Nano. 11, 1196–1203 (2016).
Zhdanov, V. P. Ostwald ripening of charged supported metal nanoparticles: Schottky model. Phys. E 71, 130–133 (2015).
Yati, I. Effects of sintering-resistance and large metal–support interface of alumina nanorod-stabilized Pt nanoparticle catalysts on the improved high temperature water gas shift reaction activity. Catal. Commun. 56, 11–16 (2014).
Gholami, R., Alyani, M. & Smith, K. Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 5, 561–594 (2015).
Liu, W. et al. Single-atom dispersed Co–Nl–C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 7, 5758–5764 (2016).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal–support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Maat, H. J. & Moscou, L. A study of the influence of platinum crystallite size on the selectivity of platinum reforming catalysts. In Proc. 3rd Int. Cong. Catal. 1277 (North Holland Publishing Company, Amsterdam, 1965).
Chandler, B. D. Strong metal–support interactions: an extra layer of complexity. Nat. Chem. 9, 108–109 (2017).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Addou, R. et al. Influence of hydroxyls on Pd atom mobility and clustering on rutile TiO2(011)-2 x 1. ACS Nano 8, 6321–6333 (2014).
Strayer, M. E. et al. Charge transfer stabilization of late transition metal oxide nanoparticles on a layered niobate support. J. Am. Chem. Soc. 137, 16216–16224 (2015).
Lu, Z. S. & Yang, Z. X. Interfacial properties of NM/CeO2(111) (NM = noble metal atoms or clusters of Pd, Pt and Rh): a first principles study. J. Phys. Condens. Matter 22, 10 (2010).
Rim, K. T. et al. Charging and chemical reactivity of gold nanoparticles and adatoms on the (111) surface of single-crystal magnetite: a scanning tunneling microscopy/spectroscopy study. J. Phys. Chem. C 113, 10198–10205 (2009).
Abbet, S. et al. Acetylene cyclotrimerization on supported size-selected Pdn clusters (1 ≤ n ≤ 30): one atom is enough! J. Am. Chem. Soc. 122, 3453–3457 (2000).
Chen, Y. Improved performance of supported single-atom catalysts via increased surface active sites. Catal. Commun. 75, 74–77 (2016).
Campbell, C. T. & Mao, Z. Chemical potential of metal atoms in supported nanoparticles: dependence upon particle size and support. ACS Catal. 7, 8460–8466 (2017).
Hastie, T., Tibshirani, R. & Friedman, J. Linear Methods for Regression (Springer, New York, 2009).
Giordano, L., Baistrocchi, M. & Pacchioni, G. Bonding of Pd, Ag, and Au atoms on MgO(100) surfaces and MgO/Mo(100) ultra-thin films: a comparative DFT study. Phys. Rev. B 72, 11 (2005).
Hinnemann, B. & Carter, E. A. Adsorption of Al, O, Hf, Y, Pt, and S atoms on α-Al2O3 (0001). J. Phys. Chem. C 111, 7105–7126 (2007).
Melnikov, V. V., Yeremeev, S. V. & Kulkova, S. E. Theoretical investigations of 3d-metal adsorption on the α-AL2O3 (0001) surface. Russ. Phys. J. 54, 704–712 (2011).
Hernández, N. C., Graciani, J., Márquez, A. & Sanz, J. F. Cu, Ag and Au atoms deposited on the α-Al2O3(0001) surface: a comparative density functional study. Surf. Sci. 575, 189–196 (2005).
Hosokawa, S., Taniguchi, M., Utani, K., Kanai, H. & Imamura, S. Affinity order among noble metals and CeO2. Appl. Catal. A 289, 115–120 (2005).
Si, R. & Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction. Angew. Chem. Int. Ed. 47, 2884–2887 (2008).
Noronha, F. B., Fendley, E. C., Soares, R. R., Alvarez, W. E. & Resasco, D. E. Correlation between catalytic activity and support reducibility in the CO2 reforming of methane over Pt/CexZr1−xO2 catalysts. Chem. Eng. J. 82, 21–31 (2001).
Szabova, L., Camellone, M. F., Huang, M., Matolin, V. & Fabris, S. Thermodynamic, electronic and structural properties of Cu/CeO2 surfaces and interfaces from first-principles DFT + U calculations. J. Chem. Phys. 133, 234705 (2010).
Murgida, G. E. & Ganduglia-Pirovano, M. V. Evidence for subsurface ordering of oxygen vacancies on the reduced CeO2(111) surface using density-functional and statistical calculations. Phys. Rev. Lett. 110, 246101 (2013).
Artiglia, L. et al. Introducing time resolution to detect Ce3+ catalytically active sites at the Pt/CeO2 interface through ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. Lett. 8, 102–108 (2017).
Eldar, Y. C. & Kutyniok, G. Compressed Sensing: Theory and Applications (Cambridge Univ. Press, Cambridge, 2012).
Ghiringhelli, L. M., Vybiral, J., Levchenko, S. V., Draxl, C. & Scheffler, M. Big data of materials science: critical role of the descriptor. Phys. Rev. Lett. 114, 105503 (2015).
Ghiringhelli, L. M. et al. Learning physical descriptors for materials science by compressed sensing. New J. Phys. 19, 023017 (2017).
Goldsmith, B. et al. Uncovering structure–property relationships of materials by subgroup discovery. New J. Phys. 19, 013031 (2017).
Pilania, G. et al. Machine learning bandgaps of double perovskites. Sci. Rep. 6, 19375 (2016).
Medasani, B. et al. Predicting defect behavior in B2 intermetallics by merging ab initio modeling and machine learning. npj Comput. Mater. 2, 1 (2016).
Oliynyk, A. O., Adutwum, L. A., Harynuk, J. J. & Mar, A. Classifying crystal structures of binary compounds AB through cluster resolution feature selection and support vector machine analysis. Chem. Mater. 28, 6672–6681 (2016).
Hong, W. T., Welsch, R. E. & Shao-Horn, Y. Descriptors of oxygen-evolution activity for oxides: a statistical evaluation. J. Phys. Chem. C 120, 78–86 (2016).
John, J. & Bloch, A. N. Quantum-defect electronegativity scale for nontransition elements. Phys. Rev. Lett. 33, 1095–1098 (1974).
Zunger, A. Systematization of the stable crystal structure of all AB-type binary compounds: a pseudopotential orbital-radii approach. Phys. Rev. B 22, 5839 (1980).
Waber, J. T. & Cromer, D. T. Orbital radii of atoms and ions. J. Chem. Phys. 42, 4116–4123 (1965).
Miedema, A. R., de Châtel, P. F. & de Boer, F. R. Cohesion in alloys—fundamentals of a semi-empirical model. Phys. B + C 100, 1–28 (1980).
Brown, I. D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model (Oxford Univ. Press, Oxford, 2002).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Kanoun, M. B., Reshak, A. H., Kanoun-Bouayed, N. & Goumri-Said, S. Evidence of Coulomb correction and spin–orbit coupling in rare-earth dioxides CeO2, PrO2 and TbO2: an ab initio study. J. Magn. Magn. Mater. 324, 1397–1405 (2012).
Mayernick, A. D. & Janik, M. J. Methane activation and oxygen vacancy formation over CeO2 and Zr, Pd substituted CeO2 surfaces. J. Phys. Chem. C 112, 14955–14964 (2008).
Krcha, M. D. & Janik, M. J. Examination of oxygen vacancy formation in Mn-doped CeO2 (111) using DFT + U and the hybrid functional HSE06. Langmuir 29, 10120–10131 (2013).
Neugebauer, J. & Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 46, 16067–16080 (1992).
Acknowledgements
This work was supported by National Science Foundation grant CHE-1505607.
Author information
Authors and Affiliations
Contributions
N.J.O. completed the DFT calculations and associated data analysis. A.S.M.J. completed the statistical learning analysis. The project idea was conceived by M.J.J. and T.P.S. All authors contributed to writing the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supporting Information
Supplementary Figures 1–13; Supplementary Tables 1–11; Supplementary Methods; Supplementary References
Supplementary Data
The values of all primary descriptors
Rights and permissions
About this article
Cite this article
O’Connor, N.J., Jonayat, A.S.M., Janik, M.J. et al. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat Catal 1, 531–539 (2018). https://doi.org/10.1038/s41929-018-0094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0094-5
This article is cited by
-
Bridging the complexity gap in computational heterogeneous catalysis with machine learning
Nature Catalysis (2023)
-
A deep learning framework to emulate density functional theory
npj Computational Materials (2023)
-
Lattice confined Ru single sites in hollow Co9S8 polyhedron triggering Co-S-Ru catalytic centers for rechargeable Zn-air battery
Nano Research (2023)
-
One-pot synthesis of toluene from methane and methanol catalyzed by GaN nanowire
Nano Research (2023)
-
Oxygen reduction electrocatalysis: From conventional to single-atomic platinum-based catalysts for proton exchange membrane fuel cells
Frontiers in Energy (2023)