Click here to download a PDF of this information.
Biologics and Biosimilars
To learn more about biologics and biosimilars, including updates on provincial policies regarding coverage of these medications, click here.
What are Biologics?
 Biologics are very specific, highly effective medicines made in living cells. They improve health in many complex conditions, including Crohn’s disease, ulcerative colitis, diabetes, rheumatoid arthritis, cancer, osteoporosis, psoriasis, human immunodeficiency virus (HIV), multiple sclerosis, growth deficiencies, and more.
Biologics are very specific, highly effective medicines made in living cells. They improve health in many complex conditions, including Crohn’s disease, ulcerative colitis, diabetes, rheumatoid arthritis, cancer, osteoporosis, psoriasis, human immunodeficiency virus (HIV), multiple sclerosis, growth deficiencies, and more.
Some examples of biologics include hormones, blood products, cytokines, growth factors, vaccines, gene and cellular therapies, fusion proteins, insulin, interferon, and monoclonal antibody (mAb) products. Patients receive biologics mainly by injection under the skin (subcutaneously) or by intravenous infusion. You cannot take these medicines orally, since the process of digestion (e.g., acidic stomach pH, digestive enzymes, and limited permeation through the gastrointestinal (GI) tract) breaks down the biologic, making it ineffective. Ongoing research into developing less-invasive or non-invasive routes for the systemic delivery of biologics that bypasses the GI tract includes via the inner cheek (buccal), through the nose (nasal), under the tongue (sublingual), inhalation into the lung (pulmonary), and through the skin (transdermal). While all these routes avoid transit through the gastrointestinal tract, each has its own strengths and weaknesses that may be optimal for specific classes of compounds. You can take some biologic vaccines currently available intra-nasally.
We have been using biologic medicines since 1796, when scientists created the first rudimentary vaccines for smallpox. Biologics have continued to evolve throughout the years into powerful medicines that have revolutionized treatment for debilitating diseases, giving patients a new chance for a better life.
What are Biosimilars?
Biosimilars are brand name products that are highly similar to an already approved brand name innovator or originator biologic but, unlike a generic drug, are not identical. To understand why biologics cannot be identical to each other, we need to look at how biologics are different from other medicines.

Small Molecule: Most medicines, such as acetylsalicylic acid or ASA (Aspirin®), are small molecule products. This means that they have simple molecular structures with low molecular weight. These small structures are easy to produce or copy. Once a patent expires, other manufacturers can make copies of small molecule drugs by reproducing the exact same active ingredient (typically a chemical) as the original product. After Health Canada approval, they can then sell this copy as a generic version. The original product and the identical generic copies are considered bioequivalent because the active part of the medicines behave the same way in the body producing equivalent levels of drug in the body. A generic manufacturer is not typically required to conduct studies in patients with specific illnesses (clinical trials) for Health Canada to approve a generic drug to treat the same conditions for which the original patented medicine has approval. However, they do require bioequivalence studies with healthy volunteers measuring the amount of drug in the body.
Biologics: By comparison, biologics are very large and have complex molecular structures, created by living cells, from specialized ingredients, using an intricate biotechnology process. It is impossible to produce an exact copy without using the exact same ingredients, the same living cell lines, and identical manufacturing conditions. In fact, it is not possible to demonstrate that a batch of any biologic – originator or biosimilar – is identical to its previous batches, but it must be within a tight range set by Health Canada. All companies must report to Health Canada any significant changes in the manufacturing process.
As with other medicines, once a patent expires for a biologic, it is legal for other manufacturers to copy the drug. However, the innovator company doesn’t have to share its patented manufacturing processes (which may include the room temperature, the type of cells that produce the biologic, and the food the cells use to grow), and since there is always variability in a live biological system, it is impossible to create an identical biosimilar. Since biosimilars are not identical, they are not generic versions of the biologic they are mimicking.
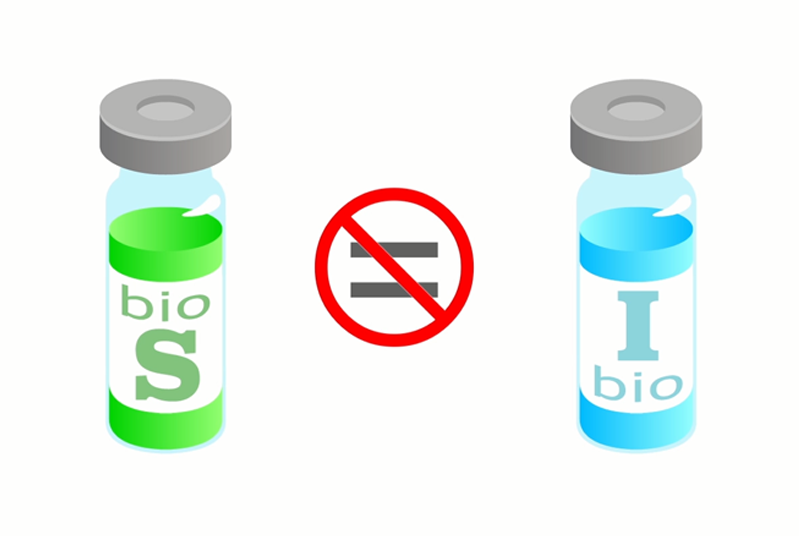
Differences: With small molecule drugs, there are often many generic versions. They all work the same and you can interchange or substitute them with each another. For example, whether you buy name brand Aspirin® or one of the many generic versions, they are the same chemical and – for the most part – perform in the same way in the body. However, with biologics, there could be many different biosimilars all referencing one drug, each with a different manufacturing process, resulting in a similar molecule to the innovator, but there are no requirements for studies or comparisons among biosimilars.
Biologic and Biosimilar Regulations in Canada
Health Canada approves biosimilars as new drugs by comparison with an innovator reference product previously authorized and marketed in Canada. As the federal authority, Health Canada states that biosimilars are not generic biologics. Health Canada only approves biosimilars for marketing in Canada when the manufacturer demonstrates that their product is of similar quality, safety, and efficacy to the original reference drug. Health Canada describes a biosimilar as a drug that has been demonstrated to be highly similar to a reference biologic drug and that there are no clinically meaningful differences in safety and efficacy between them. Clinical trials in patients (not just healthy volunteers) with at least one of the conditions the reference biologic treats are required for Health Canada to approve a biosimilar.
There are many biosimilars on the market (with more than one biosimilar version for each reference product), and many more are on the way, as the patents for originator biologics continue to expire.
Health Canada makes decisions to approve some biosimilars to treat conditions that the originator biologic has approval for, through educated assumptions by extrapolating scientific evidence. Therefore, a biosimilar might have an indication to treat a specific disease without testing in that disease. The array of indications for some biosimilars might be fewer than the array of indications for the reference product. This may be due to patent protection for specific indications, that the process of gathering the data necessary for Health Canada is ongoing, or due to a manufacturer not requesting regulatory approval for a specific indication. Different biosimilars of the same reference product might not have all of the same approved uses (known as indications).
Substitution, Interchangeability, and Non-Medical Switching
Typically, if a person has a prescription for a small molecule medication, a pharmacist may substitute a generic version without consulting or informing the patient’s physician. This is interchangeability. However, biosimilars are not interchangeable with their reference biologic in Canada. A physician may choose to switch their patient from an originator biologic to the biosimilar product if they think it will be beneficial, but a pharmacist may not change the prescription for an originator biologic to a biosimilar.
Physicians, in consultation with their patients, should make a decision about the right medication to prescribe in each unique situation, using the available clinical evidence and any relevant provincial or territorial policies.
A non-medical switch occurs when someone other than your physician says you have to change your medicine for no medical reason, typically when a government or private insurance payer will not cover your original medication and only pay for an alternative, for a number of different reasons, discussed below (Benefits of Biosimilars). Non-medical switching from originator biologics to biosimilars is arising in Canada, without the support of the Canadian Association of Gastroenterology.
To assess whether switching is a good thing, professional physician associations should develop disease-specific exclusion criteria for vulnerable patients, and governments need to monitor patients for whom they have mandated a switch policy closely, e.g., thorough data analysis and impacts on patients in conjunction with patient groups and patient registries.
Immunogenicity
Because biologics are proteins, the body can develop antibodies to them over time, which might affect how they work. This immune response, called immunogenicity, can also occur with biosimilar products, and you should discuss this with your physician before initiating therapy or switching among biologic products. Although initially there were theoretical concerns that a switch to a biosimilar may enhance the development of immunogenicity, studies have not demonstrated this to be occurring. More concern arises when a patient takes a medicine holiday and then tries to resume the same medication, as this is more likely to cause antibodies to form, so be sure you follow your prescribed medicine regime.
Naming Biosimilars
The naming of biosimilars has been a complicated issue. Typically, each version of the same small molecule medication uses the same international non-proprietary name (INN) because they are the same molecule. However, biologics aren’t identical, yet we still use the same INN for originator biologics as well as biosimilars. For example, Remicade® is the originator brand name of the INN infliximab, for which there are currently three brand name biosimilars, Inflectra®, Renfexis®, and Avsola™. Because of this, Health Canada and other similar groups internationally have looked into options for distinguishing between reference biologics and biosimilars. As of 2019, Health Canada has settled on a biosimilars naming convention that involves identifying the products by their INN in combination with their unique brand name, without the addition of a product-specific suffix. The World Health Organization is still deliberating on a global position.
Benefits of Biosimilars
Treatment options are very important for patients who have serious health conditions. With appropriate regulations in place around approval, advertising, and post-market monitoring, as well as private and public insurance coverage, products that demonstrate effective and safe treatment of conditions should be available for physicians and their patients to choose from.
The cost of pharmaceuticals, particularly biologics, is an important consideration as experts look at ways to make pharmaceutical care sustainable. Due to their complexity, biologics are costly and consume a large portion of public and private drug spending. In most cases, biosimilars are less costly than the originator biologic, given that biosimilar manufactures only need to demonstrate that their product is safe, effective, and of quality, but do not need to conduct extensive clinical trials, as the innovative manufacturer had to do, to be granted approval in one or more indications. However, at the end of a product’s patent life, most manufacturers are willing to lower their prices to compete in the biosimilar market space.
Controversy exists because some originator biologic companies have offered to match the price of the biosimilars so a patient can remain on the reference product but some government and private drug plans are refusing to allow this, even though they could have the same, or greater, savings. These issues may be due to some manufacturers being unable to publicly disclose the lower prices. The gastrointestinal community is concerned about some governments’ refusal to allow patients to stay on their original medication when finances are no longer an issue.
Conclusion
Biosimilars are not generic versions of originator biologics, but they can be affordable alternatives that work similarly to achieve similar outcomes. For patients who have not begun an originator biologic, biosimilars may be a useful lower cost alternative for physicians to prescribe. However, if the costs of biosimilars are close to the cost or the originator biologic, then the Canadian Association of Gastroenterology recommends that gastroenterologists prescribe the originator, because these have been in use for a longer period. Only a physician, in consultation with the patient, should switch a patient from an originator biologic to a biosimilar, and only if it does not adversely affect patients’ health.
Questions to ask your healthcare provider about biosimilars
Are you considering talking to your healthcare provider about biosimilars or has your healthcare provider talked to you already about biosimilars? As patient organizations, we have put together some questions that might be helpful to be part of a conversation with your healthcare provider about biosimilars and originator biologics.
- Will the biosimilar provide me with similar benefits and risks as my current biologic (if you are currently on one)? If not, what might be different? How would I know if I have an adverse reaction (to a biosimilar or biologic)?
- Can I go back to the original medication I was on if the biosimilar does not work as well for me, or if I experience different side effects?
- What is the name of the biosimilar(s) that could be taken instead of the originator biologic? (Note that medications have two names – one the manufacturer gives it, which is also called the brand name, and one that is its non-proprietary or chemical/molecule name.)
- How will I take the biosimilar? If I take it by infusion, will I need to go to a different infusion centre from where I go now? (You should note if the infusion centre is easier to get to or more difficult to get to than your current centre, and if its hours of operation align with your schedule.)
- What is the dosing schedule – will it be the same as my current biologic?
- If I administer the biosimilar myself, are there any different special storage requirements? Will I receive everything I need to administer the biosimilar?
- Is the patient support program different from the one I am currently using?
- Will I be able to get the biosimilar at the same pharmacy? (You should have the option to identify your preferred pharmacy.)
- Is there a cost difference between the originator biologic and its biosimilar(s)? What will the biosimilar cost me? What does my private drug plan cover and is there an annual or lifetime maximum drug coverage? Does my physician need to complete any forms for me to receive the originator biologic if it is covered?
When your healthcare provider starts a conversation with you about biosimilars, you may also choose to ask these additional questions along with those listed above:
- Why are you recommending this biosimilar for me?
- What if I don’t want to change my medication to a biosimilar?
- What are the consequences or options?
- What is the cost to me if I choose to stay on the originator biologic?
- Does my private healthcare plan cover any part of the originator biologic?
